Who Else Wants Tips About How To Avoid Protein Precipitation

I have tried lysis buffer without tritonx100 and with 10% glycerol to stabilize the protein.
How to avoid protein precipitation. This assumes that the protein is properly. Precipitation may be prevented by adjusting the ph and/or salt concentration, by reducing the protein concentration, or by adding a mild detergent. Could anyone suggest something in order to isolate some mg of the protein avoiding precipitation?
I used tritonx100 with the hope to solubilize the protein. I mean if its highly concentrated it would result in precipitation. What i can suggest you is that try to dilute your protein.
Commonly used concentrating methods include ultra filtration [2], reverse. But the protein precipitates in less than 12 hours even at 4°c. However, the result was the same.
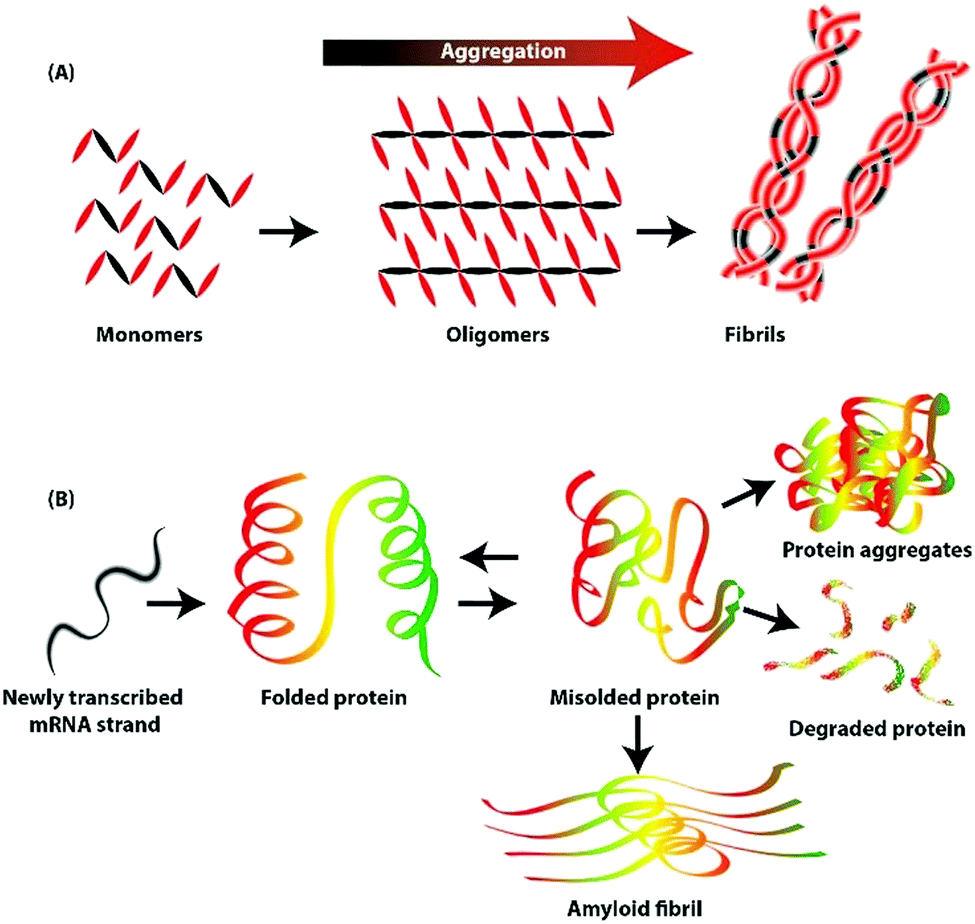
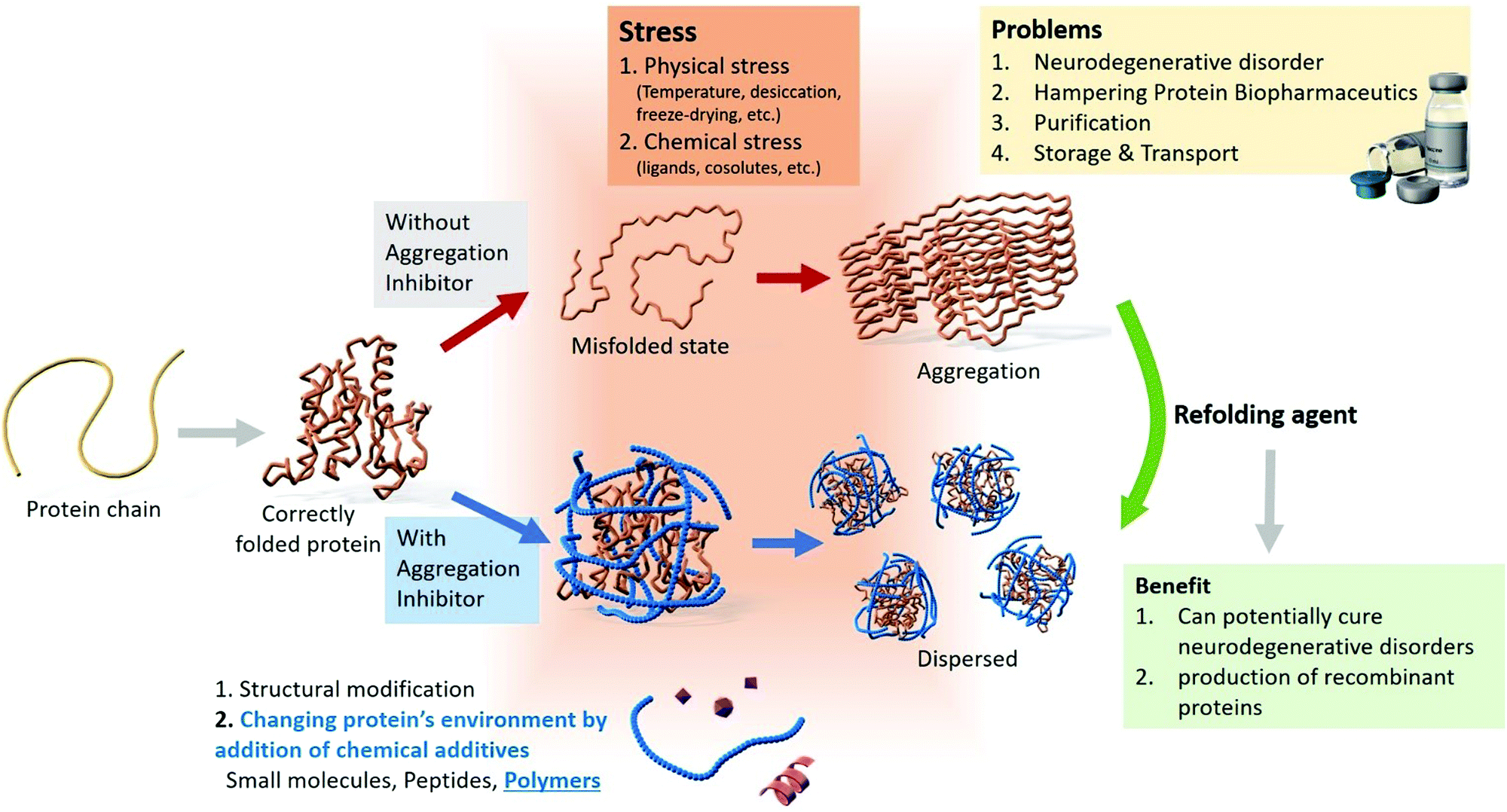
This is commonly accomplished by altering the solvent conditions and taking advantage of the changes in solubility of your protein of interest relative to those of many of the other proteins. Adding alcohol to the solution reduces the hydration of the protein, by removing water and surrounding proteins which leads to aggregation and precipitation. It is fast and your proteins may not precipitate.
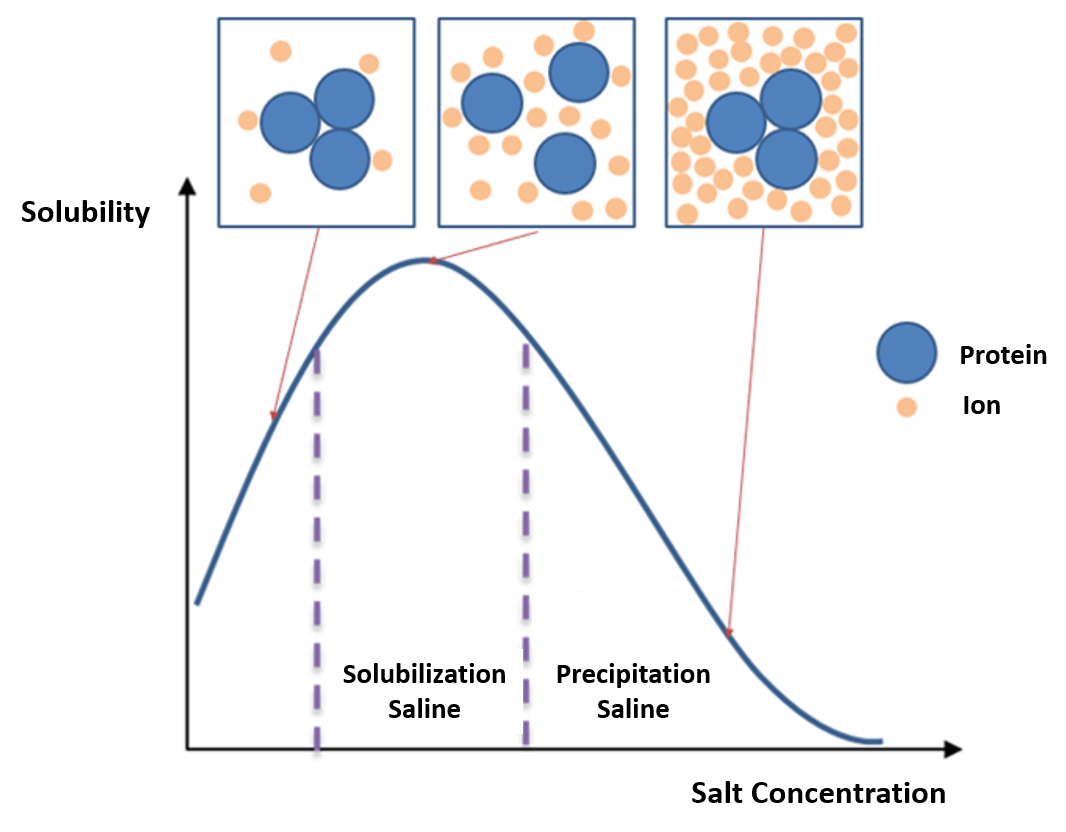
The second thing to test would be various salt concentrations. You can also check if your protein is not aggregating by any chance due to high concentration. Hope this could help u some.
Sometimes edta is not enough and you might need to add protease inhibitors. The concentration of the protein elutes is high enough (20 mg/ml) and therefore diluted with the final buffer but still it gets. Do buffer exchange and concentration just before the experiment.